
Pfizer's vaccine offers strong protection after first dose
The Food and Drug Administration's first analysis of the clinical trial data also found that the coronavirus vaccine worked well regardless of a volunteer's race, weight or age
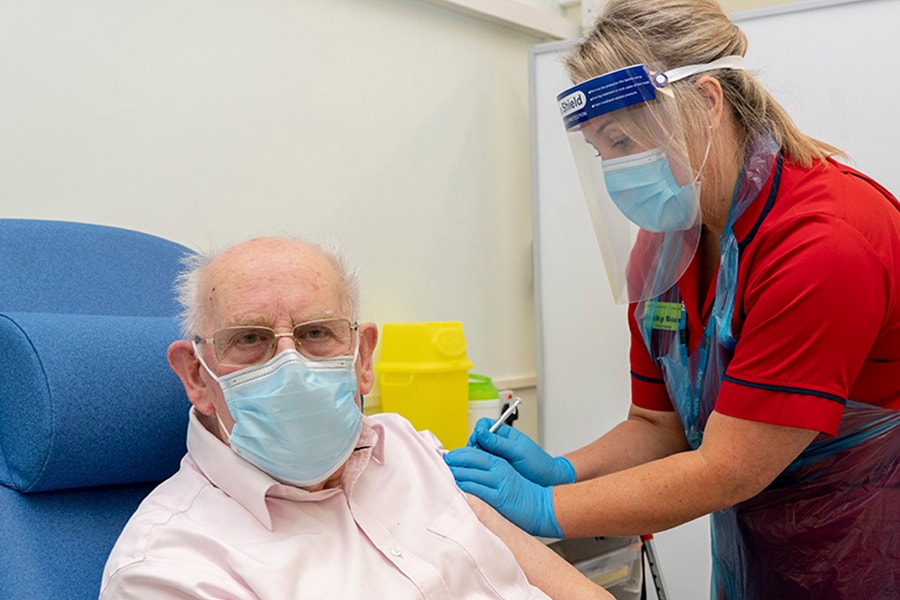 General manager of Covid Recovery, Becky Board, administers the first Pfizer-BioNTech COVID-19 vaccine in London to patient George Dyer, 90, at Croydon University Hospital, at the start of the largest ever immunisation programme in the UK's history on December 8, 2020 in London, United Kingdom.
General manager of Covid Recovery, Becky Board, administers the first Pfizer-BioNTech COVID-19 vaccine in London to patient George Dyer, 90, at Croydon University Hospital, at the start of the largest ever immunisation programme in the UK's history on December 8, 2020 in London, United Kingdom.
Image: Dan Charity - Pool/Getty Images
WASHINGTON — The coronavirus vaccine made by Pfizer and BioNTech provides strong protection against COVID-19 within about 10 days of the first dose, according to documents published Tuesday by the Food and Drug Administration before a meeting of its vaccine advisory group.
The finding is one of several significant new results featured in the briefing materials, which include more than 100 pages of data analyses from the agency and from Pfizer. Last month, Pfizer and BioNTech announced that their two-dose vaccine had an efficacy rate of 95% after two doses administered three weeks apart. The new analyses show that the protection starts kicking in far earlier.
What’s more, the vaccine worked well regardless of a volunteer’s race, weight or age. While the trial did not find any serious adverse events caused by the vaccine, many participants did experience aches, fevers and other side effects.
“This is what an A-plus report card looks like for a vaccine,” said Akiko Iwasaki, an immunologist at Yale University.
On Thursday, FDA’s vaccine advisory panel will discuss these materials in advance of a vote on whether to recommend authorization of Pfizer and BioNTech’s vaccine.
©2019 New York Times News Service




