
BioMarin: One of the world's most valuable biotechs
BioMarin became one of the world's most valuable biotechs by jettisoning costly distractions and focusing on one thing: Treating some of the rarest diseases on the planet
Before he started pre-school, little Ryan Dant could hit a baseball with the skill of a much older child. His father, a Dallas-area police lieutenant, clung to that fact when the doctors told him something was wrong. “I could throw a baseball overhand to a three-and-a-half-year-old, and he could hit it,” Dant says. “He was fine.”
But Ryan wasn’t fine. He had a rare disease called mucopolysaccharidosis I (MPS I), in which toxic, jelly-like starches built up in his body. They would severely stunt his growth, cause his organs to swell and kill him by his tenth birthday, the doctors said. Worse, the doctors told Dant, no drug company would take on the disease. Where was the profit when only a dozen American kids were born with the disorder each year?
Mark Dant refused to give up. He found a doctor who was working on an MPS I treatment, and used bake sales and golf tournaments to try to get clinical trials started. It wasn’t enough. But then a biotech startup, BioMarin, licensed the product, and the studies started. Ryan, just shy of his ninth birthday, was patient number three.
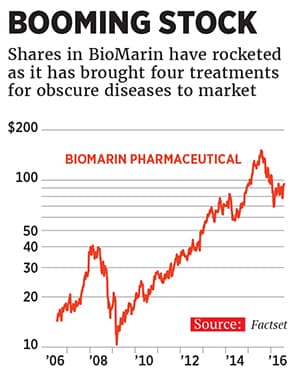
Today, BioMarin, the company that funded the testing of that drug for Ryan Dant, has annual sales of $890 million based on four approved drugs that treat just 8,000 patients worldwide. For some patients the cost of a BioMarin drug could reach $1 million a year, paid for by governments (outside the US) or insurance (inside of it). BioMarin, based in San Rafael, California, is expected to turn its first profit from continuing operations next year and has a market capitalisation of almost $15 billion, based on the medicines it sells and on hopes for more, including a gene therapy that might cure haemophilia. Shares are up by 460 percent over the past ten years.
“What we have is know-how, in terms of understanding the development and the commercialisation of drugs for ultrarare disorders,” says Jean-Jacques ‘JJ’ Bienaimé, 63, BioMarin’s chairman and chief executive.
Ryan Dant is a testament to that. Every week for 18 years, he has been hooked up to an IV to get BioMarin’s first drug, Aldurazyme. Now 28, Ryan stands 5 foot 6 inches tall, attends the University of Louisville, has a girlfriend and, in a childhood dream come true, drives a Mustang he bought using money he earned as a student equipment manager for the Southern Methodist University football team, among other jobs.
But the line connecting Aldurazyme’s approval to BioMarin’s success is anything but straight. When Bienaimé arrived at the company in 2005, Aldurazyme had been on the market for two years. Yet BioMarin looked as if it were about to go out of business.
A big part of the problem was that in 2004, previous management, doubting the potential for profits from treating rare diseases, had spent $175 million to buy a version of the steroid prednisone that was supposed to taste better so kids would take it. A generic-drug company quickly found a way around the patent, and sales of the drug amounted to just $19 million a year. Investors, enraged, started a proxy fight.
The company responded by booting the CEO and bringing in JJ Bienaimé, a Frenchman who had cut his teeth marketing the clot-buster Activase at Genentech in the late 1980s and then went on to serve as CEO of two biotechs (SangStat and Genencor) that were each sold for hundreds of millions of dollars. The idea was that he was going to dress the listing BioMarin up for sale.
But instead Bienaimé began working on a turnaround. He laid off 100 of the company’s 300 employees—basically, everyone who was supposed to be selling the tasty steroid—then handed his remaining executive team copies of Jim Collins’s book Good to Great, which posits that leaders should be humble but focussed and that confronting bad news directly is key to building a strong company.
Under the previous leadership, Emil Kakkis, the brilliant UCLA doc who had developed Aldurazyme, had been in charge not only of drug development, which he was good at, but also of sales, which he was not. Bienaimé told him to focus on the science.
“What JJ brought was a much more sensible and steady hand,” says Kakkis, now CEO of UltraGenyx, another rare-disease-focussed biotech. “He was more rational and reasonable, and not bombastic and demanding.”
At the time, BioMarin was marketing Aldurazyme in partnership with Genzyme, now part of Sanofi, in a deal that gave Genzyme half the profits (and costs) for the drug. Meanwhile, executives were negotiating another deal with Genzyme for a drug to treat a different form of MPS, called MPS VI. That deal was even worse: It would give Genzyme the international rights to the drug, Naglazyme—and most patients with MPS VI lived outside the US. Bienaimé’s decision was simple and swift: Forget it.
“It probably saved the company,” Bienaimé says. “If we had sold the rights to Genzyme, we would not be an independent company.”
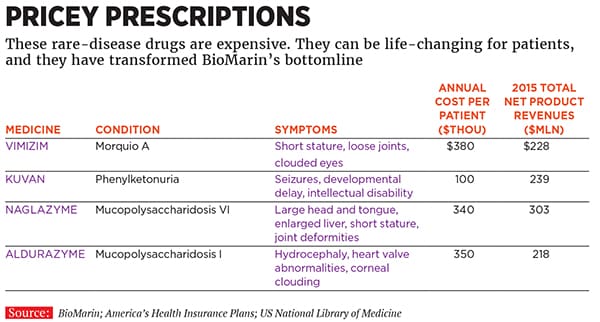
It was a crazy gamble. It meant having to build an international sales force from scratch. BioMarin’s first sales representatives in Europe operated out of internet cafes. But it worked. Approved in 2005, Naglazyme now generates annual sales of $303 million. It costs $340,000 a year for a “typical” patient, but since dosages are based on weight, it can be more expensive. In 2012, analysts at Bernstein Research estimated that for adult patients, the cost could be more than $1 million annually. BioMarin says that number is high. Now almost all of the 1,100 patients outside India and China with MPS VI take it, and 85 percent of patients live outside the US.
That was the start of a run of expensive and life-changing medicines. BioMarin’s rare-disease machine had another hit in 2007: Kuvan was approved to treat phenylketonuria (PKU), known to most people as the disease warned about on cans of Diet Coke. Patients can’t process an amino acid, phenylalanine (found in meat and Nutrasweet), and it destroys their brains. About 7,500 patients are being actively treated for PKU in clinics; treating a typical patient costs $100,000 a year. Sales in 2015: $240 million.
But BioMarin was so cash-strapped at the time Kuvan was approved that its experimental drug pipeline was dry. Kakkis, who’d shepherded its three drugs through the Food & Drug Administration, left to found a patient advocacy group (he later started another company). Bienaimé hired an old Genentech colleague, Henry Fuchs, to replace him.
Fuchs’s first drug pick was another MPS treatment, this one for MPS IVA. Called Vimizim, it is BioMarin’s biggest winner yet. Launched in 2014, the drug has already generated sales of $228 million. It costs $380,000 for a typical patient, and BioMarin thinks there are 3,000 MPS IV patients around the world; it’s identified 60 percent of them.
Who pays for these expensive drugs? In the US, mostly insurance companies or, if the patient is poor, Medicaid. In most other countries governments pay for the medicines.
Why are they willing to pay so much? Partly because these drugs do change lives, even though they are not cures. Mark Dant says Aldurazyme is not a cure for Ryan, but it is keeping him going until the science advances. “Here’s the gift of BioMarin: Ryan will be here, waiting,” he says. Dant just retired as a police officer and is now the executive director of the National MPS Society, an advocacy group.
“On my 22nd birthday, for the first time in my life, I had meat,” says Bailey Fleming, a 24-year-old Kuvan patient. Sheri Wise, 36, grew up riding horses on her parents’ Oklahoma farm even though she’s only 3 foot 4 inches in height. Vimizim made her unending skeletal pain fade. “When you grow up with pain, it just becomes a constant,” she says. “I’ve noticed that now there are a lot of times I don’t hurt at all.”
Kendra Gottsleben, 31, a Naglazyme patient in South Dakota, stands 39 inches tall and uses a wheelchair. But she says she knows it is because of BioMarin that she doesn’t need it in the house. “To make a medicine for us, I feel like it’s a miracle. Because we are so rare,” she says.
Gottsleben says that when she had insurance problems with a non-drug-related issue—a cornea transplant—she called her BioMarin customer care representative, who helped sort things out. Like most rare-disease companies, BioMarin gives money to charities that help patients when a drug is not fully covered. Because the money isn’t earmarked to pay for a particular drug, this does not run afoul of federal anti-kickback regulations.
Are insurers okay with this system? Changing it is certainly not a top priority. Patients with these diseases are so uncommon, drug companies argue, that they don’t add up to a big line item and account for, BioMarin says, only 1 percent of drug spending in the US. And unlike makers of drugs for cancer, BioMarin has “no intention of” making double-digit price increases, Bienaimé says.
But when it comes to actually trying to restrict the prices of drugs like those BioMarin sells, Steven Miller, the chief medical officer of US pharmacy benefits manager Express Scripts and a critic of high drug prices, doesn’t think insurers have much leverage.
“To be very frank, we are a price acceptor when it comes to ultra-orphans,” says Miller. “There’s no competition. It’s pharma that’s putting these prices out there. There’s nothing we can do.”
Russell Teagarden, a former senior vice president of the National Organisation for Rare Disorders and now a consultant to drug companies, thinks some pricing pressure may be imminent as rare-disease drugs get lumped in with other high-priced medicines. “There probably is a growing comfort with payers starting to [try to control] costs for rare diseases,” Teagarden says.
Given the possibility of price pressure, some of BioMarin’s next bets look risky. It is, for instance, developing a drug for children with achondroplasia, a leading cause of dwarfism. People with achondroplasia are smaller and have some skeletal problems, but unlike those with MPS, they have life expectancies that are almost normal. Bienaimé acknowledges that the drug will command a comparatively lower price.
Recent years have brought distractions. In February 2010, BioMarin spent $20 million on an experimental cancer medicine—a stretch for a rare-disease company.
It invested $100 million in the drug before changing focus and selling the drug to Medivation for $410 million and a royalty. Along the way the company got into a PR flap when a woman dying of ovarian cancer ran a social media campaign to get access to the drug. BioMarin, like most drug companies, does not give drugs to any patient who asks, both because of the cost of manufacturing the drug and the risk that bad results will hurt the drug’s chances of approval.
There was also an outright mistake: Spending $600 million to buy Kyndrisa, a treatment for muscular dystrophy, developed by a tiny biotech in Leiden, The Netherlands called Prosensa.
The drug seemed to help children with the terrible degenerative disease in an early trial but failed in a larger one. Bienaimé was preternaturally confident, telling investors he knew what he was doing and attacking a rival medicine from Sarepta, another small biotech. But both the Food and Drug Administration and European regulators rejected the drug, despite impassioned pleas from patient advocates.
“It’s true that I was very positive on Kyndrisa,” Bienaimé says.
“The Food and Drug Administration asked us to file. We had priority review, breakthrough designation. We had everything. The signals were very positive. So they do all that, and then they tell you your data were garbage.” He laughs. “Call it a mistake—fine.”
Investors, meanwhile, were getting sick of the company raking in revenue but showing no profit. Bienaimé listened and took cost-cutting measures, including stopping the cancer effort and a second rare-disease programme. BioMarin has previously recorded a profit as a result of asset sales but predicts its continuing operations will turn cash-flow-positive next year.
The most tantalising prospect is something BioMarin licensed from University College London and St Jude Children’s Research Hospital: A treatment for haemophilia. It is a gene therapy in which a virus deposits a gene in patients’ liver cells that produces a protein missing in the most common type of haemophilia. In a small clinical trial only a few months long, the treatment seems to return patients’ protein levels to almost normal. “It really is amazing. It’s just a wonderful time,” says Timothy Nichols, a haemophilia expert at the UNC School of Medicine who is not paid by BioMarin.
But Bienaimé is also facing a challenge that often causes biotech innovation to stall: Success and the size that comes with it. His 200-person startup has turned into a 2,300-person pharmaceutical company. He says it won’t happen to his company.
“I said I wanted to manage a big company,” he shrugs. “So now it is a big company.”
(This story appears in the 30 November, -0001 issue of Forbes India. To visit our Archives, click here.)





