
US to send vaccine materials and other supplies to hard-hit India, officials say
The announcement, an abrupt shift for the administration, came after Jake Sullivan, President Biden's national security adviser, held a call earlier in the day with Ajit Doval, his counterpart in India, and as India reports higher case numbers each day

A relative waits with a COVID-19 patient inside an ambulance outside a hospital in Delhi, India, April 24, 2021. (Atul Loke/The New York Times)
Under pressure from vaccine makers in India who say they need supplies to combat a surge in coronavirus cases, the Biden administration said Sunday that it had partially lifted a ban against the export of raw materials needed to make vaccines.
“The United States has identified sources of specific raw material urgently required for Indian manufacture of the Covishield vaccine that will immediately be made available for India,” Emily Horne, a spokesperson for the national security counsel, said in a statement Sunday. Covishield is the India-produced version of the AstraZeneca-Oxford vaccine.
The announcement came after Jake Sullivan, President Joe Biden’s national security adviser, held a call earlier in the day with Ajit Doval, his counterpart in India, and a day after the Indian government reported more than 346,000 new infections, a world record. Government officials in India say they are running desperately low on supplies, including oxygen and protective gear. A new variant, B.1.617, is thought to be at least partly the cause of the catastrophic rise in cases.
Previously, Biden administration officials had pushed back as pressure mounted for the United States to broaden its effort to combat the surge in India, even as horrifying images of strained hospitals and orange flames from mass cremation sites circulated around the world.
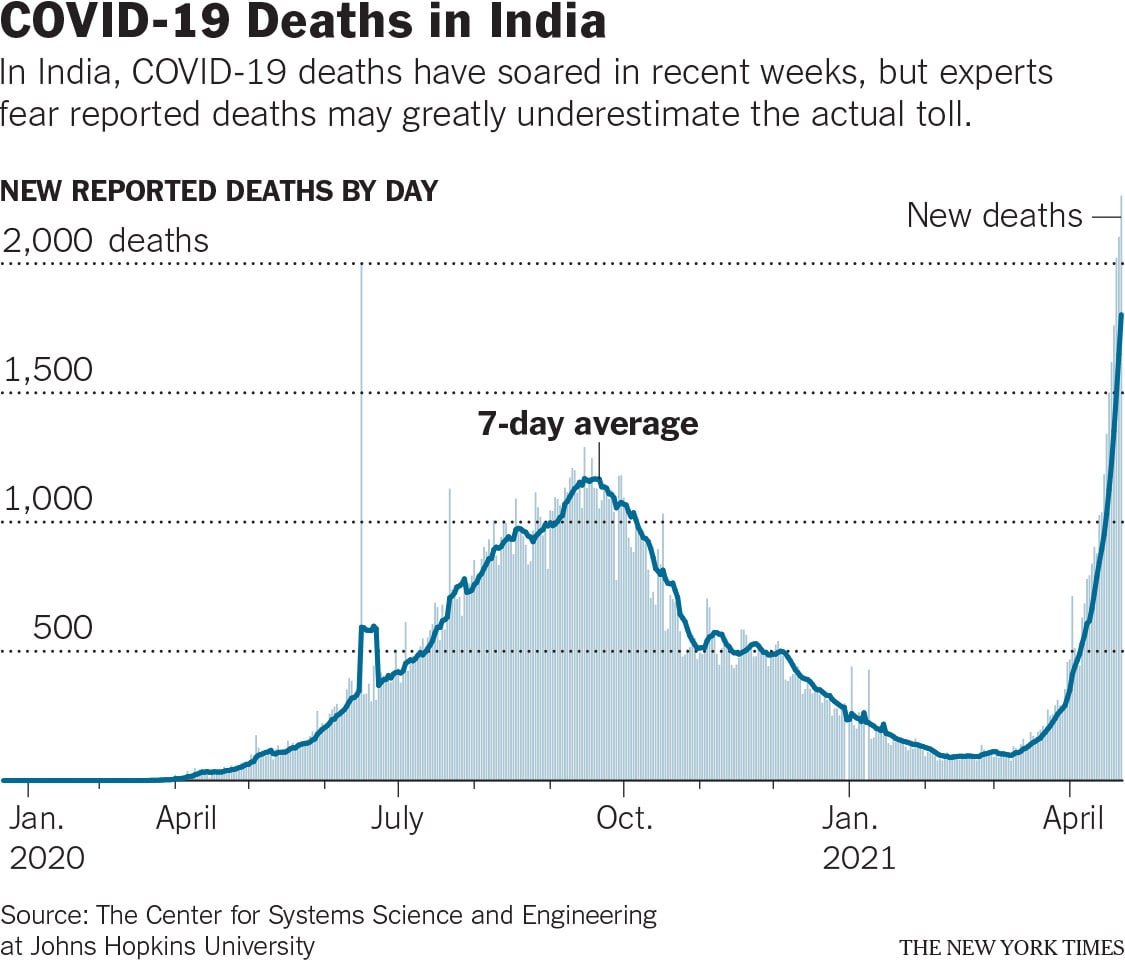
In India, COVID-19 deaths have soared in recent weeks, but experts fear reported deaths may greatly underestimate the actual toll.
©2019 New York Times News Service




