Improving access to drugs: The critical role of supply chain risk
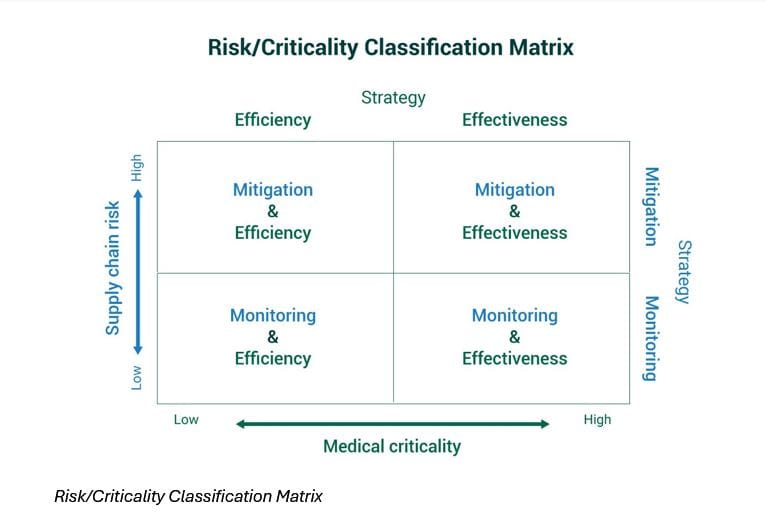
A simple way of thinking about medical criticality, supply chain risk and their interactions can lead to more sustainable solutions for drug shortages
 An increasing problem in Europe and other parts of the world, drug shortages not only pose significant challenges for healthcare professionals, but they also put public health at risk by making timely, optimal treatment impossible.
Image: Shutterstock
An increasing problem in Europe and other parts of the world, drug shortages not only pose significant challenges for healthcare professionals, but they also put public health at risk by making timely, optimal treatment impossible.
Image: Shutterstock
When pharmacists in France walked out of their counters en masse and went into the streets on 30 May, it was not just about their compensation and prospects. Their strike – the first in 10 years – surfaced a far wider problem.
While Covid-19 shone the spotlight on drug shortages, the problem exists even in the absence of large demand and supply shocks. In France, the shortage has been known to involve thousands of drugs, sometimes for months. Unreliable drug availability means pharmacists spend considerable time and effort in finding alternative solutions for patients.
An increasing problem in Europe and other parts of the world, drug shortages not only pose significant challenges for healthcare professionals, but they also put public health at risk by making timely, optimal treatment impossible. In worst-case scenarios, patient care is disrupted. In England, pharmacists have warned that drug shortages are at such critical levels that patients are at risk of immediate harm and even death. Key medicines for the treatment of type 2 diabetes, ADHD (attention-deficit/hyperactivity disorder) and epilepsy became unavailable in the UK in recent months. Outside of Europe, amoxicillin has been on the US Food and Drug Administration drug shortage database since October 2022.
Need for a structural, systemic solution
In fact, many countries have been facing structural challenges for years but have limited insight in how to resolve them. In the Netherlands, the estimated total cost of drug shortages is about EUR220 million in 2023. Drug shortage is a critical problem that needs a long-term solution.Unfortunately, most shortages are managed reactively, without accounting for the importance of supply chain risk. There is insufficient evidence of what works and little agreement among stakeholders on the causes of drug shortages. Overall, there is a lack of a systems view: stakeholders may propose interventions that may work well for a drug in the short term and in a specific country without considering the spillover effects in the long term, or on other drugs and other regions.
[This article is republished courtesy of INSEAD Knowledge, the portal to the latest business insights and views of The Business School of the World. Copyright INSEAD 2024]