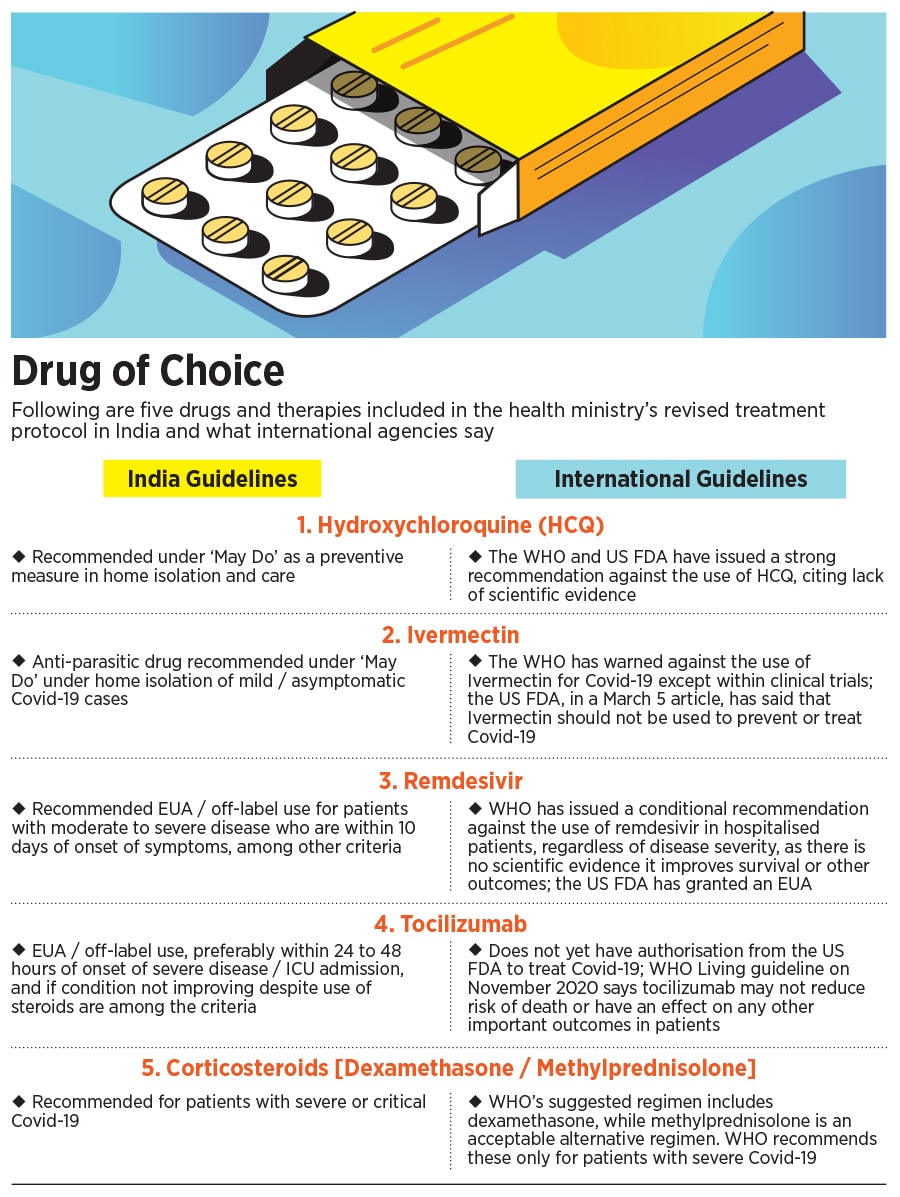
HCQ to Ivermectin: Why India has got it wrong in Covid-19 treatment
There are drugs that continue to be on the health ministry's treatment protocol despite evidence not inspiring confidence, while the regulator is approving medicines without following due scientific process, experts say
 Image: XAVIER GALIANA / AFP
Image: XAVIER GALIANA / AFP
On May 8, The Lancet, one of the world’s most respected medical journals, wrote a scathing editorial that tore into India’s vaccination strategy and the government for ignoring warnings of the second wave. It called for the implementation of a “public health response that has science at its heart”.
The very same day, India’s regulator, the Drugs Controller General of India (DCGI), green-lighted an emergency use authorisation for 2-deoxy-D-glucose (2-DG), a drug developed by the Defence Research Development Organisation (DRDO) in collaboration with Hyderabad-based pharma company Dr Reddy’s Laboratories.
The drug “helps in faster recovery of hospitalised patients and reduces supplemental oxygen dependence,” said a statement by the Ministry of Defence, adding that 2-DG will be of “immense benefit” to people suffering from Covid-19. The drug was then jointly launched on May 17 by Union Defence Minister Rajnath Singh and Union Health Minister Harsh Vardhan, who called the drug a potential game changer.
Two days later on May 19, however, the drug was left out of the revised national treatment protocol for Covid-19 that was released by the health ministry and prepared by experts at the All India Institute of Medical Sciences (AIIMS) and the Indian Council of Medical Research (ICMR). The guidelines are divided into three parts based on the severity of the Covid-19 disease. 2-DG also did not feature in the clinical management protocols updated by the health ministry on May 24. Samiran Panda, head of epidemiology and communicable diseases at ICMR, told Forbes India that experts are still studying data and evidence for the drug and that “regulatory approval is different from practice”.
By this time, however, there are several social media posts by patients requesting help to procure the drug as their doctors had prescribed it to them as part of their Covid-19 treatment. “Nobody knows why 2-DG was authorised,” says Dr Satyanarayana Mysore, HOD and consultant—pulmonology, lung transplant physician at Manipal Hospitals. “There are videos floating around on social media where the patient was administered 2-DG and recovered from Covid, which is an unbelievable claim and should be curbed.” Instead of this, he explains, what is needed is public scientific and peer-reviewed articles, or even preliminary studies, that provide evidence of the safety and efficacy of the drug.