
Yumanity and the upcoming boom in brain medicines
Advances in genetics and clinical science could lead to a flood of new treatments for depression, Alzheimer's, and even schizophrenia. That's giving hope to millions of patients-and rebooting one of the biggest markets in the history of the drug business
Tony Coles could have had any job he wanted in the drug industry. In five years at the helm of cancer drug developer Onyx Pharmaceuticals, he increased its market cap eightfold by purchasing an experimental blood cancer drug for $800 million, developing it into a big seller and flipping the whole company to Amgen for $10.4 billion in October 2013. He personally made $60 million on the deal. Biotech watchers expected him to start another cancer company or even command a drug giant like Merck or Pfizer.
Instead, Coles, 54, is using his own money to build a Cambridge, Massachusetts-based startup called Yumanity that is using yeast, the microbes that help make bread and beer, to study how misfolded proteins in the brain cause Alzheimer’s, Lou Gehrig’s disease and Parkinson’s, and to create drugs based on that knowledge. There’s already interest from Big Pharma.
Coles says he chose to attack brain diseases, not tumors, because the need is so dire and the science is so fresh.
“We’ve got 50 million people around the world who have these diseases, costing $650 billion a year, and lots of families like mine that have been affected,” says Coles. “My grandmother died of the complications of Alzheimer’s disease. I think about my own health as well.”
The modern drug business was built on brain medicines: Valium was the first blockbuster, selling two billion tablets in 1978, and Prozac defined the industry in the 1990s. But stagnant science since then led many big drug companies, including GlaxoSmithKline, Bristol-Myers Squibb and AstraZeneca, to flee neuroscience, even as an ageing population promises a dramatic surge in brain disease. In the past five years, the number of drugs being developed by large drugmakers for brain and nervous system disorders fell 50 percent to 129, according to NeuroPerspective, an industry newsletter.
But now, thanks to scientific advances such as genetic sequencing and new DNA editing technologies, the industry is in the midst of a dramatic reversal. Last year, investors poured $3.3 billion into firms that are developing drugs for brain-destroying or psychiatric illnesses, more than in any of the last ten years, says NeuroPerspective. Some big drug firms, including Johnson & Johnson, Roche and Novartis, are finding ways to reinvigorate their efforts. New medicines for severe depression, psychosis and schizophrenia could reach the market within the next few years, and treatments for Alzheimer’s, Parkinson’s and some forms of autism are a real possibility, too.
“I do think that it’s early days. There has been a fair amount of overpromising in neuroscience drug discovery,” says Ryan Watts, director of neuroscience at Roche’s Genentech division. “We have to understand there are going to be a large number of failures and little incremental victories that will start to build, and then you’ll see things cracking open.”
It will still take years for neuroscience to metamorphose from a backwater into a hotbed of innovation, but it’s happening. Mark Fishman, the head of research at Novartis, puts it bluntly: “We’re revolutionising the field.”
The history of brain drugs is based almost entirely on luck. The first antipsychotic, Thorazine, was tried on schizophrenics in the 1950s as a sedative and miraculously stopped their hallucinations. The first antidepressant, Imipramine, was an attempt at making a new antipsychotic that failed but turned out to improve mood. New blockbuster brain drugs of the past few decades—Prozac, Celexa, Zoloft, Zyprexa, Risperdal, Abilify—all mostly plumb the same basic mechanisms as the old ones: Boosting neurotransmitters like serotonin for the antidepressants; blocking the dopamine receptor D2 for antipsychotics. They differ somewhat with regard to efficacy and a lot with regard to side effects, but they operate in essentially similar ways.
For years, drug companies have been trying out new drugs that hit other chemicals without a good understanding of whether, or in whom, they’ll work.
But thanks to the revolution in our understanding of the human genome and other advances, scientists are finally starting to grasp the overwhelming complexity of illnesses that afflict the brain—and how to treat them. “Depression isn’t one disease, it’s many diseases,” says Novartis’s Fishman, who finds the new insights hopeful rather than discouraging. “Like cancer, once you understand the disease, you have hope for making drugs,” he says.
Some of the improvements are incremental. Psychiatry clinical trials often fail because placebo groups do better than they should. Part of the problem is that patients can exaggerate their symptoms to get into a study, and developing a relationship with their new doctor actually makes their symptoms seem better.
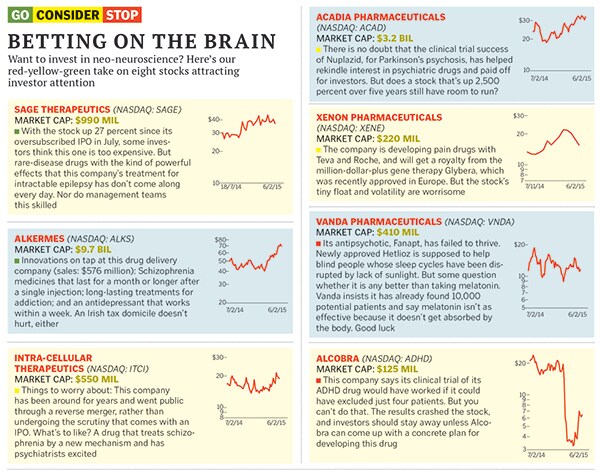
Acadia Pharmaceuticals watched its Nuplazid, for Parkinson’s psychosis, fail in a clinical trial. In 2013, it ran another study that used videoconferencing, having the same specially trained group of doctors rate the symptoms of all patients. The dramatically positive results have sent the stock up 437 percent and have shown other companies that psychiatry trials can still succeed. “We expect to become the leading neurology company in the US,” crows Acadia chief executive Uli Hacksell.
Other research has led to giant leaps forward. In 2004, researchers at the National Institutes of Health suspected that a brain receptor called N-methyl-D-aspartate, or nMda, which is key to forming memories, was also involved in depression. By luck, a group at Yale realised at the same time that ketamine, a widely used anesthetic that is also abused as a club drug called “Special K,” blocked NMDA.
The results of the first trial of ketamine in just 17 depressed people were amazing. Twelve of the patients, or 71 percent, improved, and five, or 29 percent, had their depression go into remission after getting the drug intravenously. Incredibly, their depression lifted in a matter of hours. Existing antidepressants work in only a third of patients and take weeks to have any effect. Some doctors are already giving ketamine to their patients, though the practice is controversial.
Husseini Manji, who led the nIh group doing the ketamine study, left to run neuroscience at Johnson & Johnson in 2008, where he has made a nose-spray derivative of the drug one of his top priorities; it is now entering late-stage trials. But he has competition from several other companies, including tiny Naurex of Evanston, Illinois. Ketamine causes hallucinations. Naurex makes drugs that don’t and has even tested a pill version.
Cindy Kelly, a 48-year-old mother of two, had suffered from depression on and off for 20 years until a final debilitating bout that was making it hard for her to work or relate to other people. Getting into a clinical trial for one of Naurex’s drugs changed her life.
“Within 15 minutes my symptoms were gone, and it was like magic,” she says. The effect wore off a week later, and she fought to get into a second study that would allow her to take the medicine again. She succeeded, and after several treatments, her depression is gone, seemingly for good.
“When you’re thinking, ‘Why am I even alive?’, something that takes two weeks to kick in is not helpful,” says Kelly of older depression drugs. “Something that can kick in right away so you can think clearly? That can save lives.”
Another way to find drugs that have big effects: Develop treatments for rare, terrible diseases, where creating any hope can change people’s lives. That’s the approach taken by Sage Therapeutics, a Cambridge, Massachusetts-based startup that went public in July, as it attacks a rare form of epilepsy.
Melissa Fishburn, 21, started having seizures at 14. Last November, she stiffened like a board and fell to the ground in a seizure that would not end. Doctors put her in a medically induced coma because the only hope for patients with this condition is that after resting, the patient can be brought back to consciousness and the seizure will have stopped.

But for Melissa, seizures were detectable on an electroencephalogram (EEG) even when she was fully unconscious. Doctors tried every drug they could think of, and nothing worked. “They were telling us either the seizures or the medication would end her life, one way or another,” says her father, Dale.
Melissa’s sister read about Sage’s experimental drug, Sage-547, on the internet. It blocks haywire electrical signals from jumping across nerve synapses in the brain and central nervous system. Dale mentioned it to her neurologist. Melissa, still in a coma, was flown from Springfield, Missouri, to Wichita, Kansas, to be part of a clinical trial.
After 24 hours, her EEG readings improved. Six days later, doctors started to wean her off of the drugs that kept her in a coma. A few days after that, she regained consciousness. Now she loves singing Ed Sheeran songs at karaoke. It’s not perfect: She’s never been on a date and takes 22 pills a day. But because of the Sage treatment, she’s alive.
Treatments like Sage’s are just the start of the changes scientists hope to bring about in the way we battle brain disease. Right now, for instance, patients who go to see a psychiatrist often get put on a medication based simply on what a patient tells them about how they’re feeling. When one medication doesn’t work (which is more than half the time), the doctor tries another, or a combination of drugs, based on his or her experience and gut feeling about what will work.
The reason this hit-and-miss approach fails so often, scientists are now coming to believe, is that it is based on attacking symptoms but not necessarily on what is biologically wrong with the patient.In the future, hopes Ricardo Dolmetsch, who heads neuroscience drug discovery at Novartis, when you go to a psychiatrist, she’ll consider not only your symptoms but she’ll sequence your genome as well.
That will allow her to decide on the right combination of two or three drugs to treat what is actually wrong with you. (Thomas Insel, the director of the National Institute of Mental Health, has even proposed creating a new, more genetics-based classification system for mental illness that could eventually replace the weighty bible of conditions that psychiatrists use to diagnose patients and bill insurers.)
This approach promises huge improvements in the treatment of mental illness because scientists are only now discovering just how tricky the underlying biology of mental illness can be, thanks to genetic testing.
For example, an average person has a 1-in-100 chance of developing schizophrenia. There’s a single genetic mutation called 22q11 that increases those odds to 1 in 4, but it’s rare. That’s not the only way you can develop the disease, though. You can also suffer from schizophrenia if you have lots of little mutations that add up to increase your risk.
It gets even more confusing, though, because many of those same tiny mutations that can cause schizophrenia can also lead to autism, ADHD, bipolar disorder and other mental illnesses. It’s not so much that they cause any one disease, Dolmetsch says. It’s that each mutation makes the brain’s machinery a little more “flaky,” in his words. Depending on these variations and when in a brain’s development they occur, different mental disorders result.
To deal with this terrifying complexity, drug companies are embracing new technologies—including brain cells created in the laboratory expressly for research purposes—that allow them to test medicines with unprecedented speed and precision. “That’s the single most important piece here,” says Stevin Zorn, the head of research at Lundbeck, the $2 billion (sales) Danish maker of antidepressants. “We’re starting to see a light that a lot of companies are starting to follow.”
Nobody is embracing these technologies more fiercely than Dolmetsch. In 2007, he was not a pharmaceutical executive but a rising assistant professor at Stanford, studying ivory tower questions about how nerve cells communicate. Then his son was diagnosed with autism.
He gave up all the grants that were paying for his laboratory and started pursuing what are called induced pluripotent stem cells, cells that can be made from a flake of skin or a drop of blood and turned into any tissue in the body—including brain cells.
At first, Dolmetsch focussed on a rare disease, Timothy Syndrome, that causes both autism and heart problems. He was interested in learning about his son but became fascinated by drug discovery. “There’s very little hope for these people. I really became committed to the cause,” he says.
He started one project to make induced pluripotent stem cells at the pioneering Allen Institute for Brain Science in Seattle, which is funded by Microsoft billionaire Paul Allen. But after coming to Novartis to talk about collaboration, it became clear that Novartis was a better fit. The drug giant was willing to give the 44-year-old neophyte a blank slate.
The reason the stem-cell-based “brains in a dish” are a big deal is that these cells have huge advantages over mice brains, which researchers traditionally use to test drugs. Mice are not people—not even close. We’re separated from Mickey by 60 million years of evolution. Mice with 22q11 mutations never get schizophrenia. Mice don’t get Alzheimer’s, either.
So far, Dolmetsch and his Novartis team have made hundreds of batches of these brains in a dish in a sprawling laboratory in Cambridge, Massachusetts, using samples taken from people with mental illness. For common diseases like schizophrenia and depression, there will be a tedious process of turning genes on and off to see what they do. But for some rare diseases, Dolmetsch merely screens Novartis’s library of drugs against the cells to see if he can make them normal.
The results are already promising. After only two years, Novartis is planning to enter two medicines in clinical trials as a result of the new screening technique. That’s made him, like many others in the field, boundlessly hopeful and energetic about what comes next. “I want to restart neuroscience,” Dolmetsch says. The reboot is under way.
(This story appears in the 30 November, -0001 issue of Forbes India. To visit our Archives, click here.)






