
Citing the Delta variant, Pfizer will pursue booster shots and a new vaccine
A booster given six months after the second dose of the vaccine increases the potency of antibodies against the original virus and the beta variant by five- to tenfold, Pfizer and BioNTech said
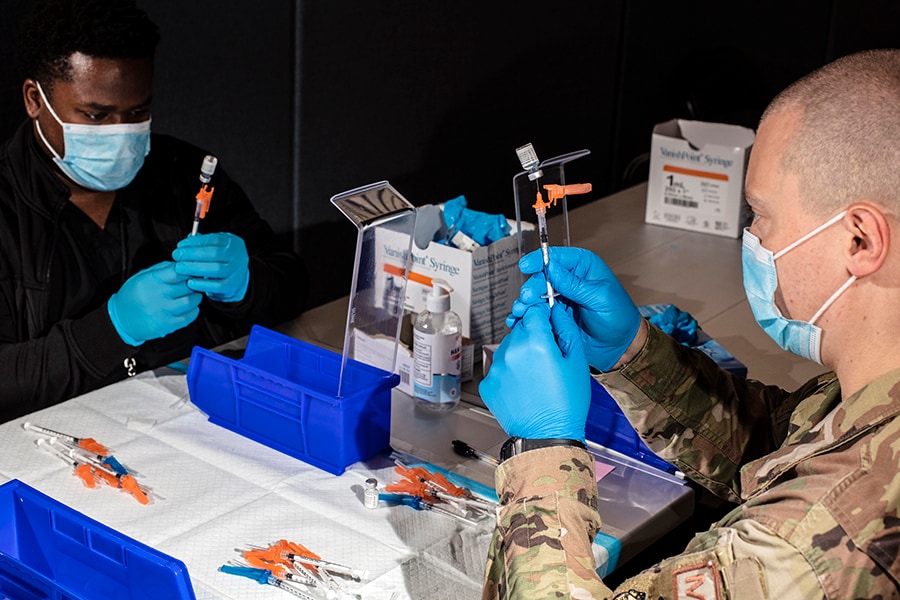 A member of the U.S. military and an employee of the New Jersey Institute of Technology prepared doses of the Pfizer-BioNTech COVID-19 vaccine at an immunization site in Newark, N.J., June 19, 2021. The Delta variant of the coronavirus can evade antibodies that target certain parts of the virus, according to a new study published on Thursday, July 8, 2021, in Nature.
A member of the U.S. military and an employee of the New Jersey Institute of Technology prepared doses of the Pfizer-BioNTech COVID-19 vaccine at an immunization site in Newark, N.J., June 19, 2021. The Delta variant of the coronavirus can evade antibodies that target certain parts of the virus, according to a new study published on Thursday, July 8, 2021, in Nature.
Image: Bryan Anselm/The New York Times
Pfizer and BioNTech announced Thursday that they were developing a version of the coronavirus vaccine that targets delta, a highly contagious variant that has spread to nearly 100 countries. The companies expect to begin clinical trials of the vaccine in August.
Pfizer and BioNTech also reported promising results from studies of people who received a third dose of the original vaccine. A booster given six months after the second dose of the vaccine increases the potency of antibodies against the original virus and the beta variant by five- to tenfold, the companies said.
Vaccine efficacy may decline six months after immunization, the companies said in a news release, and booster doses may be needed to fend off virus variants.
The data have not been published, nor peer-reviewed. The vaccine makers said they expected to submit their findings to the Food and Drug Administration in the coming weeks, a step toward gaining authorization for booster shots.
But the companies’ assertions contradict other research, and several experts pushed back against the claim that boosters will be needed.
©2019 New York Times News Service




