
Ennoventure's fight against fake drugs
Technology company Ennoventure applied cryptography to build an authentication tool that has been granted two US patents. The solution can benefit the pharma industry in combating counterfeiters
 (From left) Ennoventure CTO Shalini V Nair and CEO Padmakumar Nair at the company’s office in Bengaluru
(From left) Ennoventure CTO Shalini V Nair and CEO Padmakumar Nair at the company’s office in Bengaluru Image: Hemant Mishra For Forbes India
Shalini V Nair was reading a research paper on cryptography and steganography methods used by the Central Intelligence Agency to send images on internet messengers when her mobile phone rang. Steganography hides data by embedding it into an audio, video, image or text file. It protects secret or sensitive data from malicious attacks. Cryptography protects information and communication by using codes, so that only those for whom the information is intended can read and process it.
“I was running my own startup and doing AI (artificial intelligence)-related work for a client in Australia,” says the 44-year-old engineer of that day in Bengaluru in October 2017. It was Paddy or Padmakumar Nair, 41, at the other end, calling from Dubai. They knew each other since their days as engineering students.
In his circle, Paddy is known for inquiring and scoping out details to define a problem, before posing it to 3-4 people. Paddy filled Nair in on the underbelly of large industries (counterfeit products) and the opportunity to build a software tool that can verify the authenticity of product packages, thereby preventing proliferation of counterfeit goods. “We need a solution that can be patented, unique and non-disruptive of processes at client’s site. The solution has to be invisible (covert). And we need a way for only a smartphone to authenticate—not expensive scanners.” Paddy told her. Over that week, Nair was coding.
In a few months, she co-founded Ennoventure with Paddy, and Joseph G Harb, who lives in the US. By August 2018, Shalini—who is also CTO—and Ennoventure applied for two patents in the US. “One patent is for the full flow—how we verify the authenticity of products using the mobile phone to scan labels which contain our invisible cryptographic code,” she explains. “The other is specific to what we do: The profiling for design and how we optimise our cryptographic code for a particular design.” One patent was granted on November 2018, and the second on February 2019.
Problem of Counterfeits
The solution developed by the company can be applied in many industries, but Ennoventure has begun with pharmaceuticals. The authentication tool can be accessed by packaging departments of pharma companies easily as it is a SaaS (software as a service) tool that resides on cloud servers. It has started to get commercialised.
The packaging departments of pharma companies can upload an artwork (package design) on which the Ennoventure algorithm encrypts information proprietary to the client. This is invisible to the eye. The encrypted package design can then be printed on the packages of medicines made by Ennoventure’s clients. After a batch of medicines leaves the factory, a distributor or retailer can download an app and use their smartphone to check the veracity of medicines.
The threat of counterfeit or falsified medicines is a stated risk factor in the annual reports of most pharma companies. The 2018 annual report of the $53-billion (by revenue) Novartis warns shareholders that falsified medicines are visually indistinguishable from genuine ones and require a forensic authentication process of the packaging and/or the actual medicine to ascertain their falsified nature and likely impact on patient safety.
“Thefts of our genuine products from warehouses or plants, or while in-transit, which are then not properly stored and are later sold through unauthorised channels, could adversely impact patient safety, our reputation and business,” the report states. “There is a direct financial loss when, for example, falsified medicines replace sales of genuine medicines, or genuine medicines are recalled following discovery of falsified products.”
The World Health Organization (WHO) states that counterfeiting can apply to both branded and generic medicines, and counterfeit products may include products with the correct or wrong ingredients, without active ingredients, with insufficient active ingredients or with fake packaging.
In November 2013, Interpol (The International Criminal Police Organization) launched a three-year Pharmaceutical Crime Programme with 29 drug makers, including GSK, to build on its Medical Product Counterfeiting and Pharmaceutical Crime unit. In the same year, the US Senate brought to life the Drug Quality and Security Act to strengthen the prescription drug supply chain and save families from the menace of counterfeit drugs.
The European Union wasn’t far behind. An EU Falsified Medicines Directive has set up the first “end-to-end verification system” for drugs across the world. The deadline for implementation of the requirements across Europe was February 9, 2019.
As developed markets made the transition, so did India because of its considerable pharmaceutical exports. Track-and-trace became a buzzphrase, putting the onus on pharma companies to use barcodes to identify medicines.
“In India, instances of counterfeiting of drugs and nutraceuticals are quite high,” says Shwetasree Majumder, managing partner of Fidus Law Chambers, an intellectual property law firm in Noida. “Counterfeit drugs pose serious health risks for patients, and damage a pharma company’s reputation, impact its sales and make it vulnerable to probing from the drug authorities.”
 Pharmacists have the onus to ensure that the drugs they sell are legal under relevant drug laws
Pharmacists have the onus to ensure that the drugs they sell are legal under relevant drug lawsImage: Hemant Mishra For Forbes India
It can open up two types of disputes, she explains. First, trademark infringement. “Other companies using deceptively similar marks for the same or different drugs.
This causes consumer confusion and poses serious health risks,” Majumder says. Second, imitating of trade-dress. “Other companies may copy the packaging and ‘look’ of a particular medicine, which also leads to consumer confusion. This becomes even more serious as several patients may be illiterate or unable to read a particular language.”
Even as national regulators, WHO and agencies like IRACM (Institute of Research Against Counterfeit Medicines) have made it a discussion point, incidents go unrecorded when counterfeit drugs are seized because of the damage they do to the reputation of multinationals.
Forbes India spoke to a former legal head of an Indian pharma multinational and a senior manager (strategy) of a multinational giant. Neither was willing to come on record. “It’s a much bigger issue for smaller pharma companies that have a fair bit of rural business. Companies with business around urban conglomerates don’t really face this problem,” says the strategy head. “Counterfeit drugs are segment-specific and don’t affect more specialised products, say for cardiovascular diseases, anti-cancers etc. A lot of counterfeits are for mass-market products like pain killers, antibiotics.”
The former legal head says combating counterfeit drugs became important for two reasons: To prevent pilferage by making packaging hard to replicate and because the US made track-and-trace packaging compulsory. “Special ‘track and trace’ packaging helps understand whether something was produced by a manufacturer or not, when it was produced, which batch, who it was sold to, etc,” he says. “You can track the whole supply chain with track-and-trace packing.”

Costs for Pharma
Padmakumar, who is CEO of Ennoventure, learnt about the anti-counterfeiting opportunity when he met Harb, 42, at MIT Sloan School of Management during their executive education programme in Cambridge, Massachusetts. Harb worked with Johnson & Johnson (J&J) in Africa then, and had experienced how counterfeit drugs were leading to financial losses.
“Joseph picked up a lotion stock that had been replenished by a different supplier. But he couldn’t tell if the lotion was genuine or fake,” Paddy recalls. “He sent it to J&J’s labs in the US where they found that the lotions were counterfeit.”
Typically, pharmaceutical companies train staffers to send drugs suspected to be counterfeit to the headquarters for verification. Second, ‘mystery shoppers’ purchase suspected items as civilians, and send them to the company lab. Third, government regulators also organise random checks. “If a government body finds even one product to be fake, the entire batch is taken out of the market,” says Paddy. The officials check for falsified packaging and drugs manufactured for another geographic market being sold in their jurisdiction.
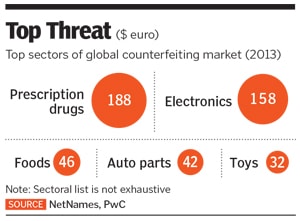
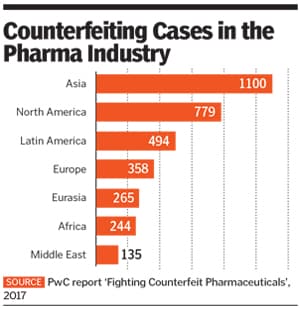
According to a June 2017 PwC report ‘Fighting Counterfeit Pharmaceuticals’, counterfeit pharmaceuticals form a €188 billion ($200 billion) market, making it the largest segment of the fraudulent goods sold around the world.
If a regulator finds counterfeit drugs, the official sends a letter to the company asking for the stock to be cleared. “At this stage, a company can spend as much as $150,000 per investigation,” says Paddy. At best, an investigation agency can find out which country the falsified stock has come from, and through which port.
Paddy wants to solve this and use the SaaS path to scale Ennoventure. And also allow pharmaceutical companies to have a proactive approach. For example, governments insist on serialisation, asking every pharma company to have a unique number for each product. This can be expensive, which has pushed pharma companies to invest in technologies to track their stock. With blockchain-based technologies, every time a batch moves from a factory to the warehouse, it gets scanned. There is a batch number for each set of medicines that is on the move.
But this can be replicated by counterfeiters too. “Once a counterfeiter figures out the unique batch number based on paper trail, it’s only a matter of imitating the same batch number on a 2D barcode,” says Paddy. The fake drugs are packaged with the batch number. “So now, you have the same number of fake units as genuine medicines with a common batch number. How will a distributor know which one is genuine?” he asks.
This is where a cryptographic approach will prove cost-efficient to deploy across a range of product packages, and as a potent method to combat counterfeiters. “Ours is an authentication tool rather than straight-out track and trace. It’s more AI and cryptography,” says Shalini. “When we do extend it to tracking, it will be entirely different. The US law mandates that every drug shipped to the US needs a tracking number or barcode with specific details prominently displayed.”
With a 2D barcode for tracking, pharmaceutical factories have to alter the workflow because they have to vary the process based on product packaging and size—to imprint the barcode. That won’t be necessary with Ennoventure because the design can be provided by the manufacturer for the encryption to take place. It can’t be replicated because the information is not visible. It’s a cryptographic code, with a biometric reference (fingerprint of any company official), which ensures it cannot be copied.
A distributor or sub-distributor can check it with an app. “When you take a picture using the app, the AI system helps check if Ennoventure is working with the company,” says Paddy. If it is, the validation process happens. “Then, the font and design get checked in line with the original artwork. Finally, there’s a check of the decoded information.” The encrypted approach can be rendered even on medicine strips, which none of the existing solutions can do. “2D barcodes are hard to design on strips. We check at a pixel level,” says Paddy.
Ennoventure has begun to deploy the solution with the Chordia Group’s Medopharm, and with some of the leading pharmaceutical and FMCG companies in India. “The biggest loophole in today’s technology is that there is no way for an end consumer to figure out if a drug is real or fake,” says Paddy.
In the pharma value chain, there is a medical store or pharmacy at the ground level. Above pharmacies, there is a stockist or distributor, and then the C&F (clearing and forwarding) agencies who get the stock from factories. “The C&F layer has morphed into a warehouse-like facility after the Goods & Services Tax has been introduced. They are more like master-distributors,” says Mayur Shetty, director of Keweenaw Health Solutions, which specialises in stocking and supply of anti-cancer drugs.
In India, counterfeiting is rampant when distributors or customers buy medicines without a bill. “Purchases with a bill help in traceability, as long as the party issuing the bill pays back taxes due. But there are cases where rogue parties have sold counterfeit drugs after issuing bills, and then folded up their business because the counterfeit drugs have made them their money,” says Shetty.
Mass-market or popular drugs get counterfeited, but even big pharma brands are not safe. “Being a big brand allows counterfeiters to get customers’ buy-in more easily. Cardiovascular and diabetic molecules are often counterfeited,” says Shetty. That’s why traceability checks are essential at the retail level. “The onus is on pharmacists to ensure that the drugs they sell are legal under relevant drug laws, and not infringing third party IP rights,” says Majumder of Fidus Law Chambers. “Under the Trademarks Act, there is no difference in the liability of a manufacture of infringing drugs and the distributor or pharmacist selling those products.”
Ennoventure is betting on presence at the retail stage too. With scale and marketing, a SaaS product can pave the way for end-customers at pharmacies to check if the medicines are real or not. That’ll happen by using a smartphone app to check the packaging.
(This story appears in the 30 November, -0001 issue of Forbes India. To visit our Archives, click here.)




