
How India is pulling off the world's largest Covid-19 vaccination programme
As health care and frontline workers start getting inoculated from January 16, Forbes India looks at how states are gearing up to implement last-mile vaccination, where the private sector is chipping in, and the fears, hesitancy or confidence beneficiaries have regarding the vaccine shot
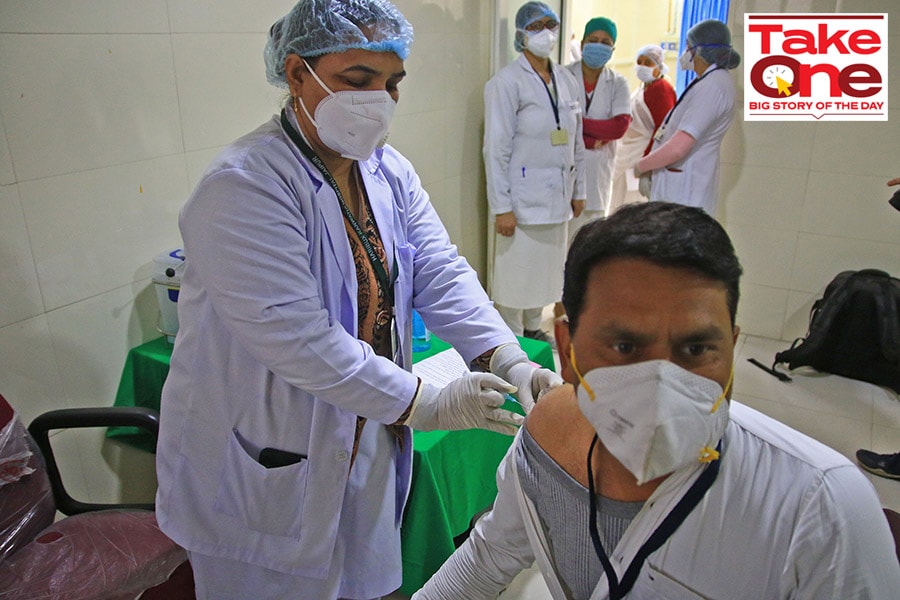 Image: Vishal Bhatnagar/NurPhoto via Getty Images
Image: Vishal Bhatnagar/NurPhoto via Getty Images
The world’s largest Covid-19 vaccination programme will begin on Saturday, January 16.
At 10:30 AM on January 16, Prime Minister Narendra Modi will officially launch the first phase of Covid-19 vaccination, involving a vaccine developed by AstraZeneca and the indigenously developed vaccine from Bharat Biotech. The phased rollouts will initially vaccinate ten million health care and 20 million frontline workers, followed by 270 million senior citizens and people with co-morbidities like diabetes, hypertension, organ transplants etc. In all, 300 million people are going to be inoculated by July this year.
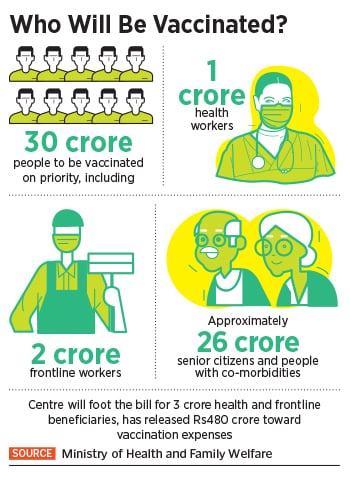 That’s roughly a little more than 20 percent of the country’s 1.3 billion population. “Prime Minister Narendra Modi will launch the pan-India rollout of Covid-19 vaccination drive on 16th January, 2021 at 10:30 AM via video conferencing,” a statement from the Indian prime minister’s office said.
That’s roughly a little more than 20 percent of the country’s 1.3 billion population. “Prime Minister Narendra Modi will launch the pan-India rollout of Covid-19 vaccination drive on 16th January, 2021 at 10:30 AM via video conferencing,” a statement from the Indian prime minister’s office said.
The vaccination programme will be held over 3,000 session sites across the country, and around 100 beneficiaries will be vaccinated at each session site on the inaugural day. India is currently the world’s second worst-hit Covid-19 country with over 10.52 million cases. Of this, over 213,000 cases are currently active.
“This will be the world’s largest vaccination programme covering the entire length and breadth of the country,” the prime minister’s office said.



 On January 13, the Chhatrapati Shivaji Maharaj International Airport (CSMIA) saw the transportation of a total of 227 boxes, containing approximately 2,72,400 doses of the vaccine, to be delivered to over 20 destinations across the country, including Agartala, Cochin, Raipur, Jammu, Srinagar, Gorakhpur, Dehradun, Jabalpur, Trivandrum and Indore. A spokesperson for the Delhi International Airport Limited (DIAL), told Forbes India in an email that India’s busiest airport with an annual cargo handling capacity if 1.8 million metric tonnes is “working with other stakeholders in the supply chain, including pharma companies, airlines, forwarders and temperature-controlled transport service providers for efficient, reliable and uninterrupted services for end users”.
On January 13, the Chhatrapati Shivaji Maharaj International Airport (CSMIA) saw the transportation of a total of 227 boxes, containing approximately 2,72,400 doses of the vaccine, to be delivered to over 20 destinations across the country, including Agartala, Cochin, Raipur, Jammu, Srinagar, Gorakhpur, Dehradun, Jabalpur, Trivandrum and Indore. A spokesperson for the Delhi International Airport Limited (DIAL), told Forbes India in an email that India’s busiest airport with an annual cargo handling capacity if 1.8 million metric tonnes is “working with other stakeholders in the supply chain, including pharma companies, airlines, forwarders and temperature-controlled transport service providers for efficient, reliable and uninterrupted services for end users”.
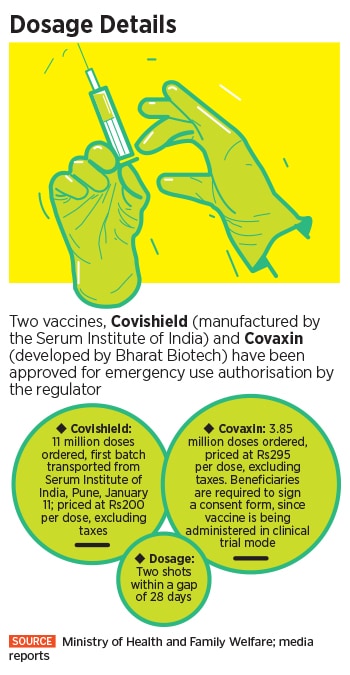 On January 13, the second consignment of 1.47 lakh doses arrived at the cold storage facility in Belgaum, taking the total number of vaccine doses to be administered in Karnataka to 7.94 lakh. Out of these, for immediate inoculation, around 15,730 doses are allocated for central government health care workers in Karnataka, 7.75 lakh doses for state health care workers and around 2,580 doses for Armed Forces Medical Services.
On January 13, the second consignment of 1.47 lakh doses arrived at the cold storage facility in Belgaum, taking the total number of vaccine doses to be administered in Karnataka to 7.94 lakh. Out of these, for immediate inoculation, around 15,730 doses are allocated for central government health care workers in Karnataka, 7.75 lakh doses for state health care workers and around 2,580 doses for Armed Forces Medical Services.
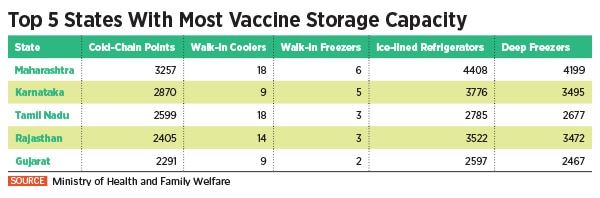
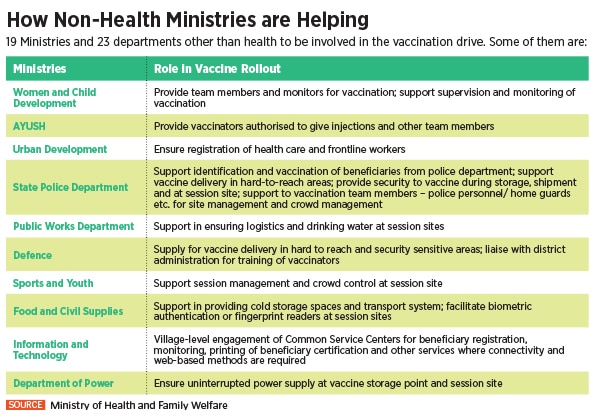
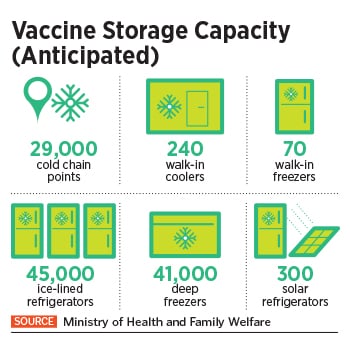
 To undertake this Herculean task, the Indian government is piggybacking on its existing immunisation infrastructure including 29,000 cold chain points, 240 walk-in coolers, 70 walk-in freezers, 45,000 ice-lined refrigerators, 41,000 deep freezers and 300 solar refrigerators. The private sector, meanwhile, expects a bigger role as the government looks at the second phase of vaccination.
To undertake this Herculean task, the Indian government is piggybacking on its existing immunisation infrastructure including 29,000 cold chain points, 240 walk-in coolers, 70 walk-in freezers, 45,000 ice-lined refrigerators, 41,000 deep freezers and 300 solar refrigerators. The private sector, meanwhile, expects a bigger role as the government looks at the second phase of vaccination.
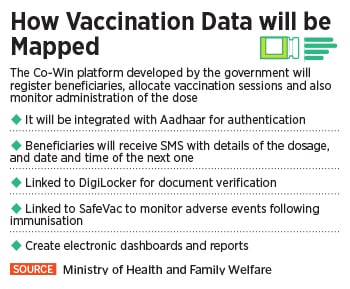 “Vaccination for Covid-19 is voluntary,” India’s health ministry had said earlier. “However, it is advisable to receive the complete schedule of the vaccine for protecting oneself against this disease and also to limit the spread to close contacts including family members, friends, relatives and co-workers.”
“Vaccination for Covid-19 is voluntary,” India’s health ministry had said earlier. “However, it is advisable to receive the complete schedule of the vaccine for protecting oneself against this disease and also to limit the spread to close contacts including family members, friends, relatives and co-workers.”




