
Inside India's Covid-19 vaccine trials: The volunteers' story
Volunteers who took the jab give Forbes India a peek into their apprehensions and the aftermath
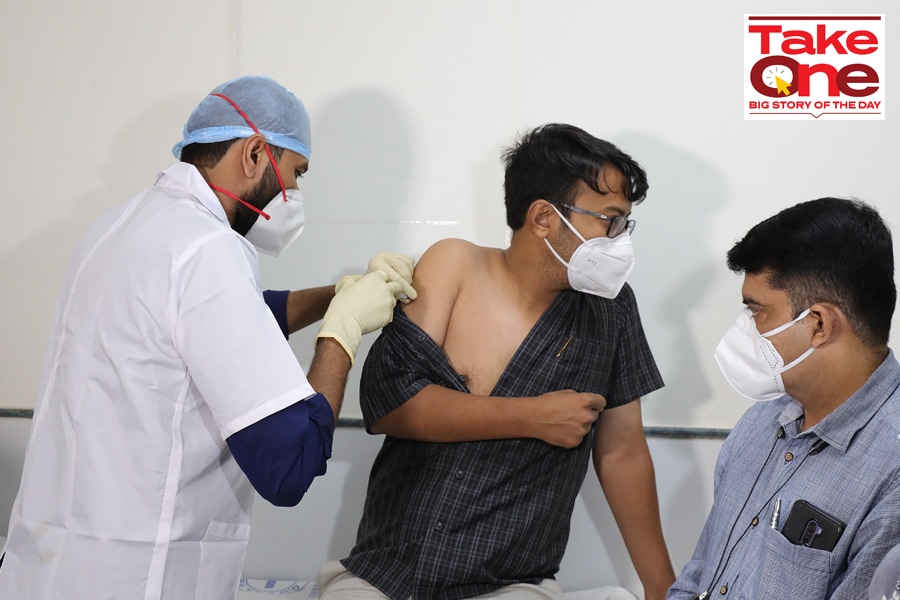
A volunteer gets a dose of Covaxin Covid-19 vaccine during a clinical trial at a civil hospital in Ahmedabad
On the morning of August 7, the mother-daughter duo of Hema and Chhavi Damani, 51 and 26 respectively, decided to twin in their T-shirts, trousers and masks. A short prayer followed before they headed for the Zydus Hospital in Ahmedabad for a “noble cause”—volunteer for the Phase 2 trials of ZyCoV-D, one of the possible Covid vaccines developed by pharmaceutical major Zydus Cadila.
“My husband is a physician at the hospital,” says Hema, a homemaker. “One day, he came home and informed us that Zydus Cadila is going to start the second round of human trials at the hospital. My daughter and I asked him if we could be a part of it.” Both the mother and the daughter didn’t have any second thoughts in putting their hands up as they knew that getting volunteers would be key to running successful trials.
A day before they got the jab, Hema and Chhavi were taken to the hospital for a screening, a blood profile and RT-PCR tests to ensure they didn’t have any antibodies already. Trial participants are expected to have normal blood pressure and sugar levels and pregnant women and children are barred from the exercise.
Once vetted, the doctor-in-charge sat them down to discuss the road ahead as well as hand over the eight-page Informed Consent Document that explained in detail the vaccine, possible side-effects, the number of visits required, the reimbursements they will receive for hospital visits, etc. They were also told that participants will be monitored for the next six months (187 days) for side-effects. “There was an initial apprehension of sorts, but once I understood the procedure in details, I wasn’t too scared,” adds Hema.








